F.D.A. Moves to Speed Approvals for Cheaper Copycat Drugs
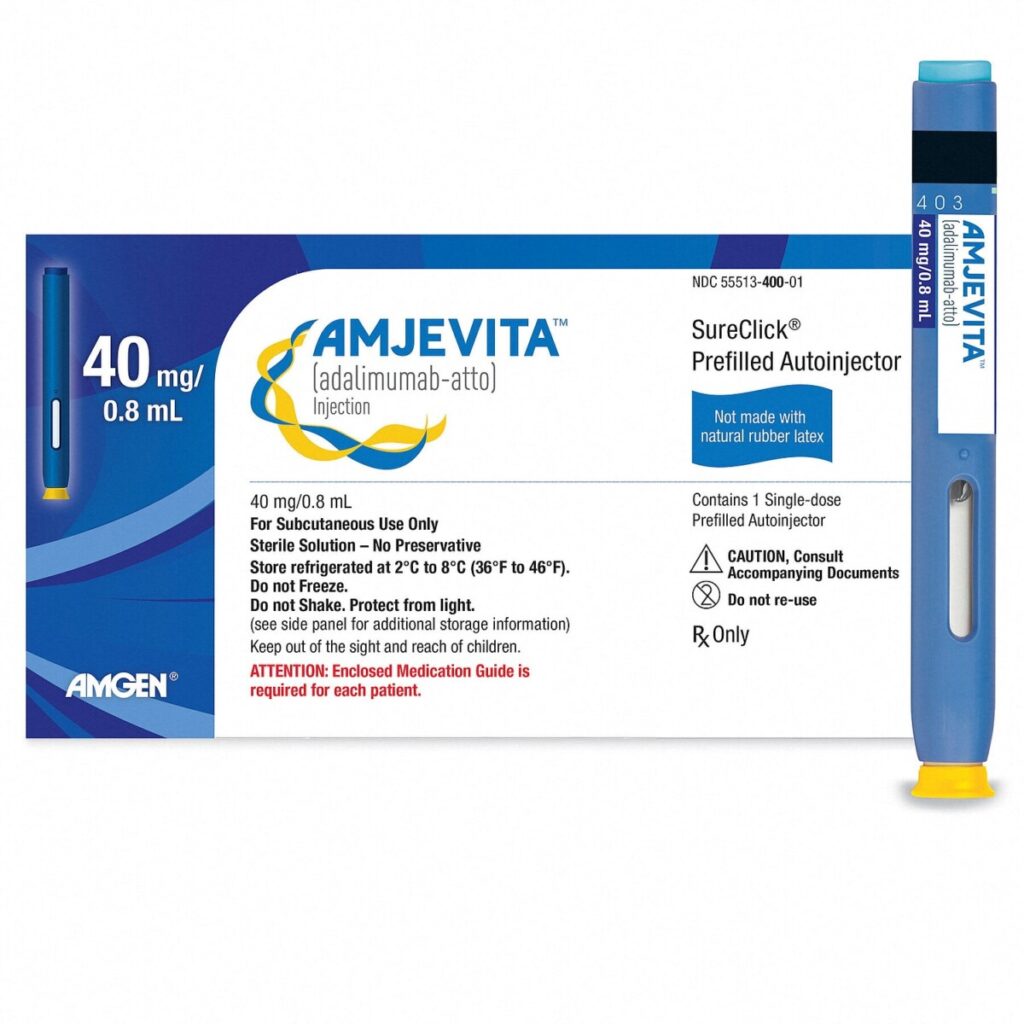
Amjevita, a biosimilar to the widely prescribed arthritis medication Humira, represents a significant development in the pharmaceutical landscape, particularly in the realm of biologic drugs. Humira, which has been a cornerstone treatment for conditions such as rheumatoid arthritis, psoriasis, and Crohn’s disease, generated billions in revenue for its manufacturer, AbbVie, making it one of the best-selling drugs in history. However, as Humira’s patent protections began to expire, a wave of biosimilars like Amjevita emerged, aiming to provide more affordable alternatives for patients. Approved by the FDA in 2016 and launched in 2023, Amjevita is part of a growing trend that has seen dozens of biosimilar drugs enter the market over the past decade, reflecting a broader push towards increasing competition and reducing healthcare costs.
Biosimilars, which are biologic medical products highly similar to already approved reference products, offer the potential for significant savings in treatment costs. For example, Humira’s list price is approximately $6,000 per year, while Amjevita is expected to be priced lower, making it more accessible for patients who struggle with the financial burden of chronic disease management. The introduction of Amjevita not only benefits patients financially but also enhances treatment options. With the increasing availability of biosimilars, healthcare providers can offer patients a wider range of therapeutic choices, potentially improving adherence to treatment regimens and overall patient outcomes.
Moreover, the rise of biosimilars like Amjevita is reshaping the pharmaceutical market dynamics, prompting original manufacturers to adapt their strategies. AbbVie, for instance, has responded by launching its own line of biosimilars and implementing patient assistance programs to maintain its market share. The competition introduced by biosimilars is expected to drive innovation and lower prices across the board, ultimately benefiting consumers. As the market continues to evolve, the impact of biosimilars on healthcare costs and patient access to essential medications will be closely monitored, highlighting the importance of these developments in the ongoing conversation about affordable healthcare.
Related articles:
– Link 1
– Link 2
Amjevita, a copycat version of the blockbuster arthritis medicine Humira, is one of dozens of biosimilar drugs that have hit the market in the past decade.
Eric
Eric is a seasoned journalist covering Health news.