Neutrogena Recalls Makeup Wipes Over Bacterial Contamination Concerns
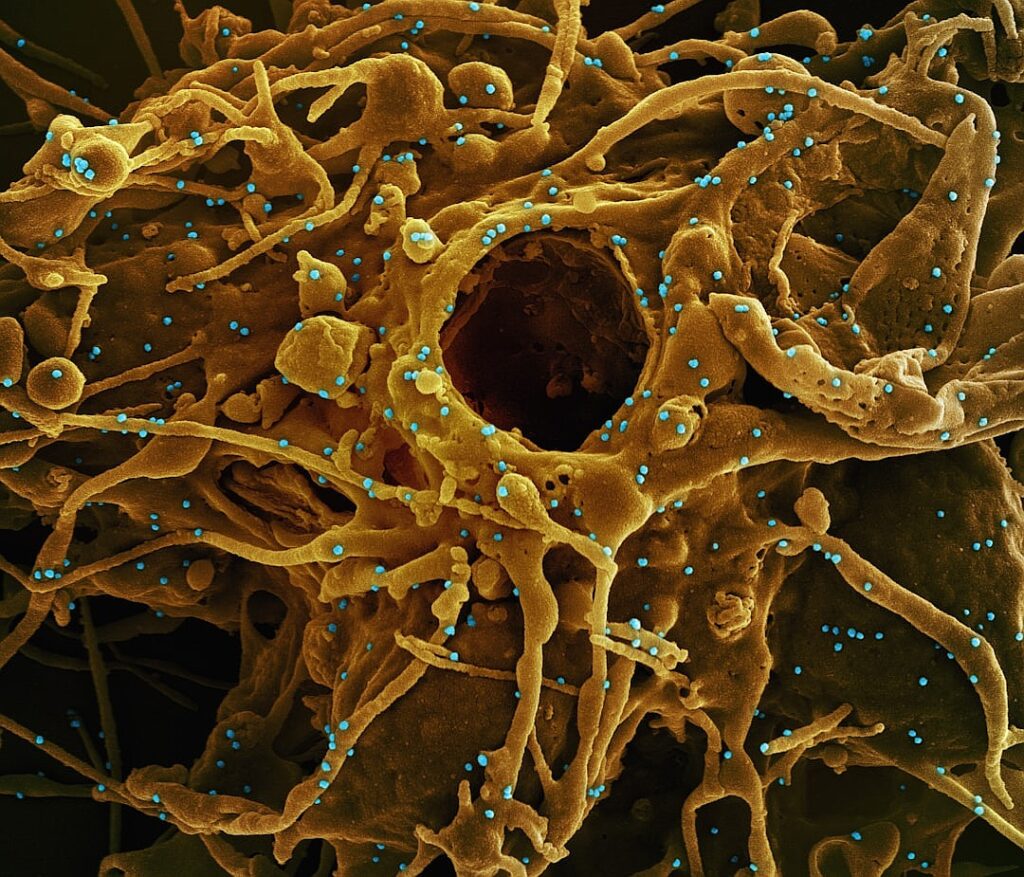
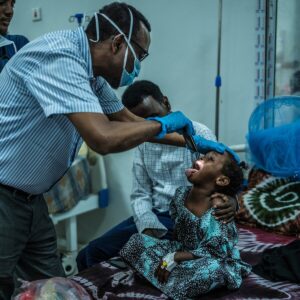
In a recent health alert, the Food and Drug Administration (FDA) announced a recall of certain wipes that were distributed across several states, including Florida, Georgia, South Carolina, and Texas. The recall stems from concerns regarding potential contamination that could pose health risks to consumers. While the specific details of the contamination have not been disclosed, the FDA emphasizes the importance of consumer safety and urges individuals who may have purchased these products to discontinue use immediately.
The affected wipes, which were widely available in retail stores and online, are often used for personal hygiene and household cleaning. The recall is particularly significant given the heightened focus on sanitation and cleanliness in the wake of the COVID-19 pandemic. Consumers are advised to check their cabinets and dispose of any wipes that fall under the recall specifications. The FDA has provided guidance on how to identify the recalled products, including specific lot numbers and expiration dates, to aid consumers in recognizing potentially hazardous items.
This recall serves as a reminder of the crucial role regulatory bodies play in monitoring product safety and protecting public health. In recent years, there have been several recalls of consumer products due to contamination issues, underscoring the necessity for vigilance among both manufacturers and consumers. As the FDA continues to monitor the situation, they encourage anyone who has experienced adverse effects from using the recalled wipes to report their experiences. This proactive approach not only helps to safeguard public health but also fosters greater transparency and accountability within the consumer goods industry.
Related articles:
– Link 1
– Link 2
The recalled wipes were distributed in Florida, Georgia, South Carolina and Texas, according to the Food and Drug Administration.
Eric
Eric is a seasoned journalist covering Health news.