Drug With a 30-Year Monopoly Is Target of State-Level Push to Curb Prices
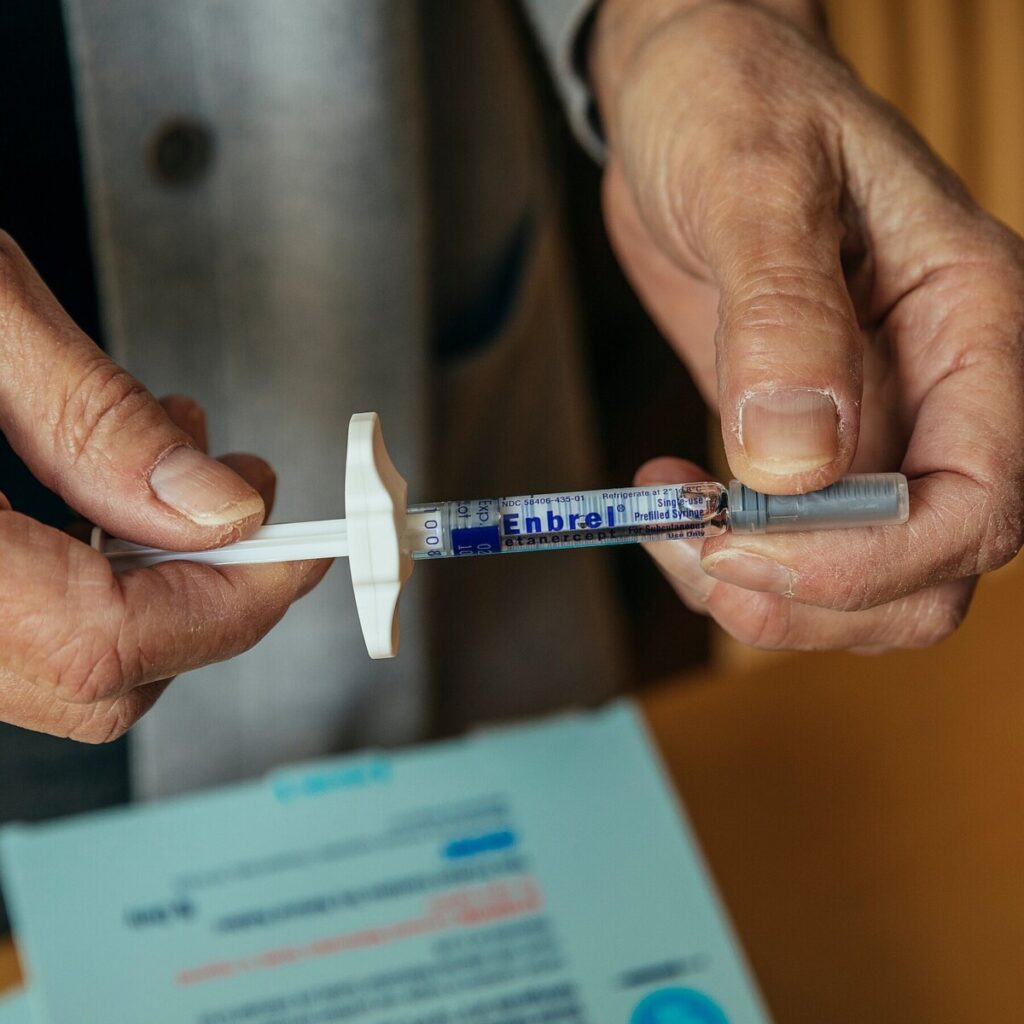
Enbrel, a groundbreaking medication that has transformed the treatment landscape for autoimmune conditions, has been a staple in the medical community since its introduction nearly 30 years ago. As a once-a-week injection, Enbrel (etanercept) is primarily used to manage conditions such as rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. By inhibiting tumor necrosis factor (TNF), a substance in the body that contributes to inflammation, Enbrel effectively alleviates symptoms and improves the quality of life for millions of patients. Its launch marked a significant advancement in the treatment of autoimmune diseases, offering a new hope to individuals struggling with chronic pain and debilitating symptoms.
The impact of Enbrel extends beyond its clinical effectiveness; it has also played a crucial role in shaping the pharmaceutical industry’s approach to biologic therapies. With the rise of biologic medications, Enbrel set a precedent for how these treatments could be developed and administered, paving the way for a new class of therapies that target specific pathways in the immune system. Over the years, numerous studies have demonstrated Enbrel’s efficacy and safety, solidifying its position as a leading choice among healthcare providers. However, the medication’s journey has not been without controversy. As patents expired and biosimilars entered the market, discussions around pricing and access to these essential medications have intensified, highlighting the ongoing challenges patients face in affording their treatments.
As Enbrel approaches its 30th anniversary, it remains a vital option for those living with autoimmune disorders. The ongoing evolution of treatment options, alongside the challenges of healthcare accessibility and affordability, underscores the importance of continued innovation in this field. With a growing emphasis on patient-centered care, the future of autoimmune disease management will likely hinge on balancing effective treatment options like Enbrel with equitable access for all patients. As we reflect on Enbrel’s legacy, it serves as a reminder of the progress made in the fight against autoimmune diseases and the work that still lies ahead to ensure that all patients receive the care they need.
Related articles:
– Link 1
– Link 2
Enbrel, a once-a-week injection used to treat a variety of autoimmune conditions, first arrived on the market nearly 30 years ago.
Eric
Eric is a seasoned journalist covering Health news.