Drug With a 30-Year Monopoly Is Target of State-Level Push to Curb Prices
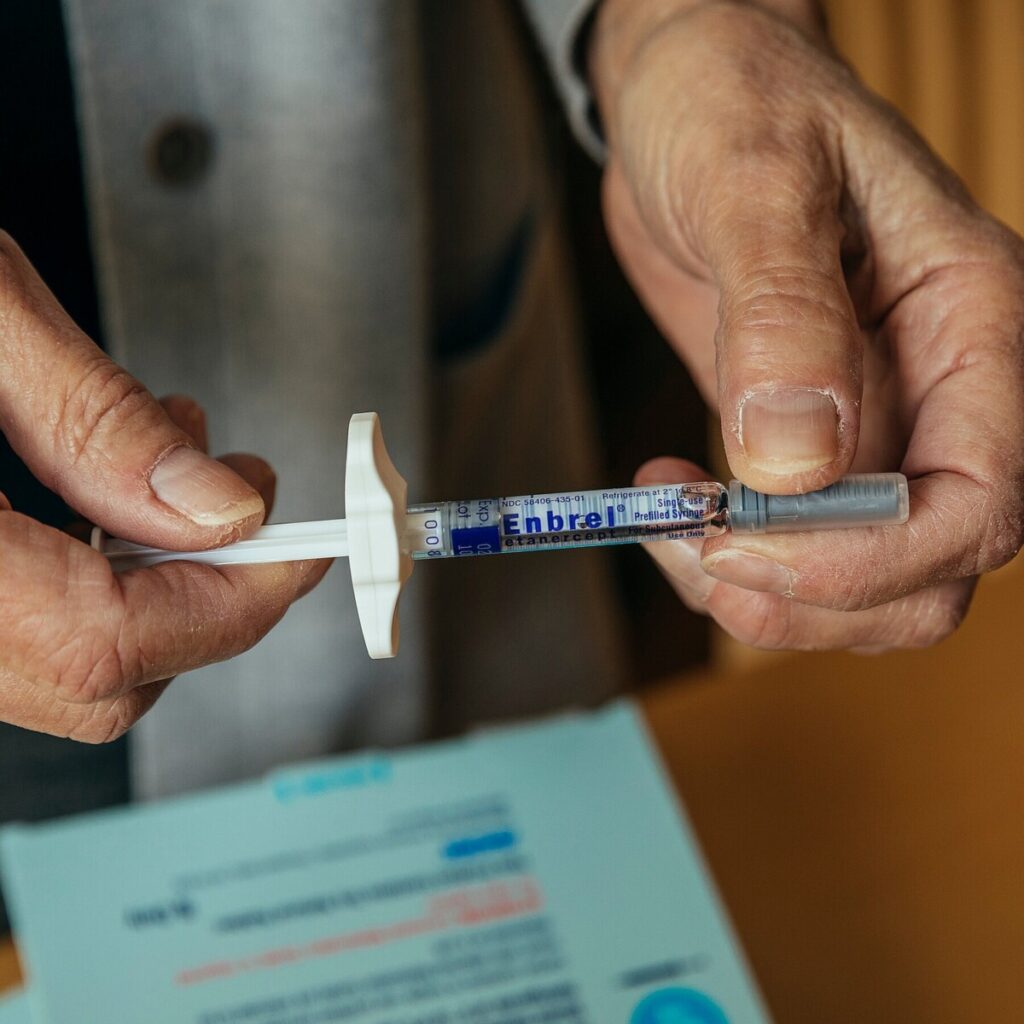
Enbrel, a groundbreaking medication that has transformed the treatment landscape for autoimmune diseases, has been a staple in healthcare since its introduction nearly 30 years ago. Approved by the FDA in 1998, this once-a-week injection has been a game-changer for patients suffering from conditions such as rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Enbrel works by inhibiting tumor necrosis factor (TNF), a substance in the body that leads to inflammation and is a key player in the pathophysiology of these diseases. By targeting TNF, Enbrel helps reduce inflammation, alleviate pain, and improve overall quality of life for many patients.
Over the decades, Enbrel has not only provided relief to millions but has also sparked discussions about the pricing and accessibility of biologic therapies. Initially developed by Immunex, the drug has had a significant impact on the pharmaceutical market, generating billions in revenue and becoming one of the top-selling drugs in the world. However, its high cost has raised concerns among patients and healthcare providers alike. With annual treatment costs reaching upwards of $60,000, many patients struggle with insurance coverage and out-of-pocket expenses. This situation has led to calls for more affordable options and greater transparency in drug pricing. As the patent for Enbrel nears expiration, the potential for biosimilars—more affordable versions of biologic drugs—has become a focal point in discussions about the future of autoimmune disease treatment.
In recent years, the conversation surrounding Enbrel has also included its long-term effects and the need for ongoing patient support. While many experience significant improvement, some patients report challenges with side effects or inadequate responses to the medication. This has prompted healthcare professionals to advocate for personalized treatment plans that consider individual patient needs and preferences. As the healthcare landscape continues to evolve, the legacy of Enbrel remains significant, highlighting the delicate balance between innovation, accessibility, and patient-centered care in the realm of chronic illness management.
Related articles:
– Link 1
– Link 2
Enbrel, a once-a-week injection used to treat a variety of autoimmune conditions, first arrived on the market nearly 30 years ago.
Eric
Eric is a seasoned journalist covering Health news.