Drug With a 30-Year Monopoly Is Target of State-Level Push to Curb Prices
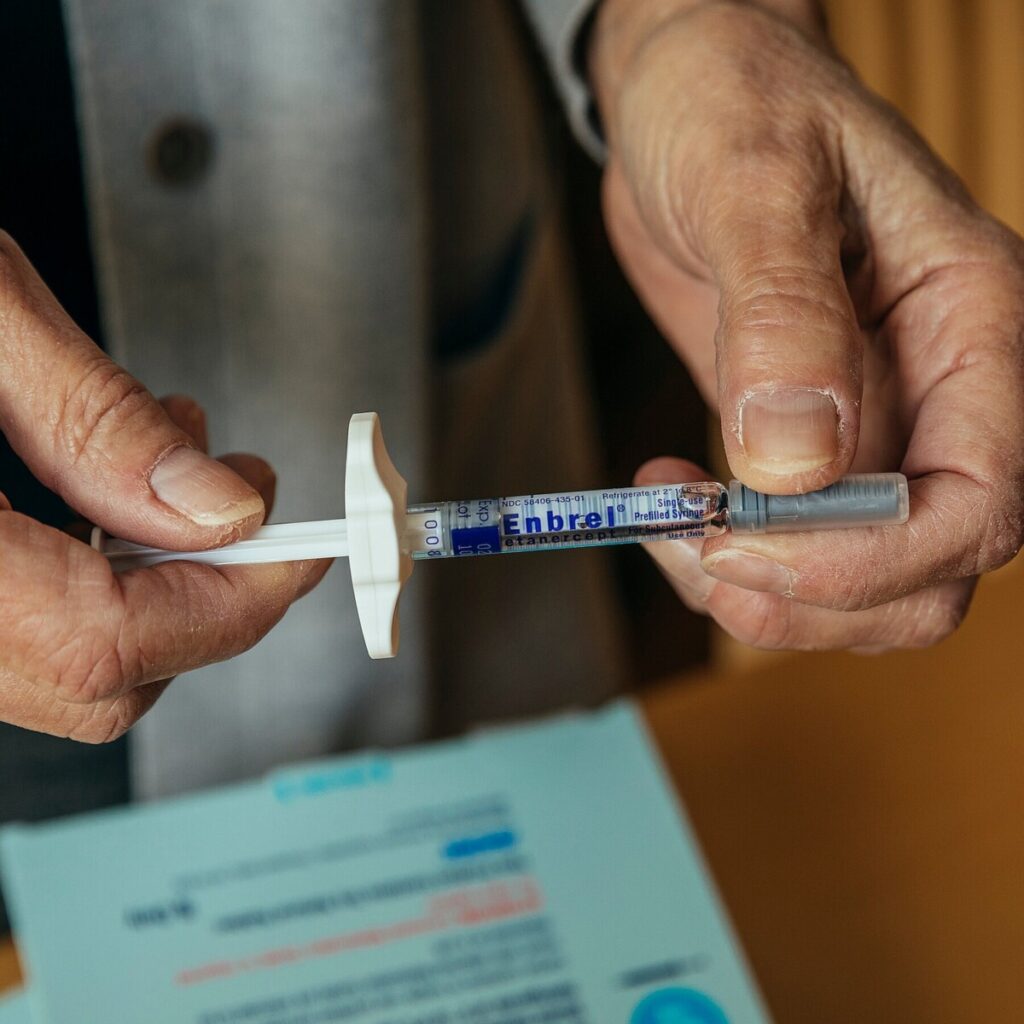
Enbrel, a groundbreaking medication for autoimmune conditions, has been a staple in treatment regimens since its introduction nearly 30 years ago. Approved by the FDA in 1998, Enbrel (etanercept) is a biologic drug that functions by inhibiting tumor necrosis factor (TNF), a substance in the body that leads to inflammation and is implicated in diseases such as rheumatoid arthritis, psoriasis, and ankylosing spondylitis. This once-a-week injection has provided relief and improved the quality of life for millions of patients by effectively reducing inflammation and preventing disease progression. The drug’s long-standing presence in the market highlights its significance in the evolution of treatment options for chronic autoimmune diseases.
Despite its success, the journey of Enbrel has not been without challenges. The medication has faced scrutiny over its high cost, which has sparked debates about drug pricing and accessibility in the healthcare system. As the patent on Enbrel has expired, biosimilars—biologically similar products—have begun to emerge, offering patients potentially more affordable alternatives. However, the transition to these biosimilars raises questions about their efficacy and safety compared to the original drug. In addition, ongoing discussions about health insurance coverage and the financial burden on patients underscore the complexities surrounding access to essential medications.
As Enbrel continues to be a pivotal treatment option for those with autoimmune conditions, its legacy serves as a reminder of the advancements in medical science and the ongoing need for equitable healthcare solutions. The drug’s impact on patient lives is profound, but it also reflects broader issues within the pharmaceutical industry, including the balance between innovation, affordability, and accessibility. As we look to the future, the development of new therapies and the introduction of biosimilars may reshape the landscape of treatment for autoimmune diseases, ensuring that patients have access to effective care without prohibitive costs.
Related articles:
– Link 1
– Link 2
Enbrel, a once-a-week injection used to treat a variety of autoimmune conditions, first arrived on the market nearly 30 years ago.
Eric
Eric is a seasoned journalist covering Health news.